FDA Issues Guidance for Clinical Research & Product Development
SILVER SPRING, M.D. – The Federal Drug Administration’s (FDA) Center for Drug Evaluation and Research issued its final nonbinding recommendations for clinical cannabis research this week. The eleven-page guidance document is intended to outline the department’s thinking on several issues regarding the development of human drugs containing cannabis or cannabis-derived compounds, sources of research materials, and considerations of control status under the Controlled Substances Act.
The document, “Cannabis and Cannabis-Derived Compounds: Quality Considerations for Clinical Research,” specifically deals with cannabis-related products intended to diagnose, cure, mitigate, prevent, or treat a disease, or any non-food products intended to affect the structure or any function of the human body. The FDA considers a cannabis product a drug when it’s “marketed with a claim of therapeutic benefit, or with any other disease-related claim,” as defined in section 201(g) of the Federal Food, Drug, and Cosmetic Act (FD&C Act). However, the guidance does not address synthetic versions of naturally occurring substances that are currently regulated like other fully synthetic drugs, such as dronabinol, which obtained FDA approval in 1985.
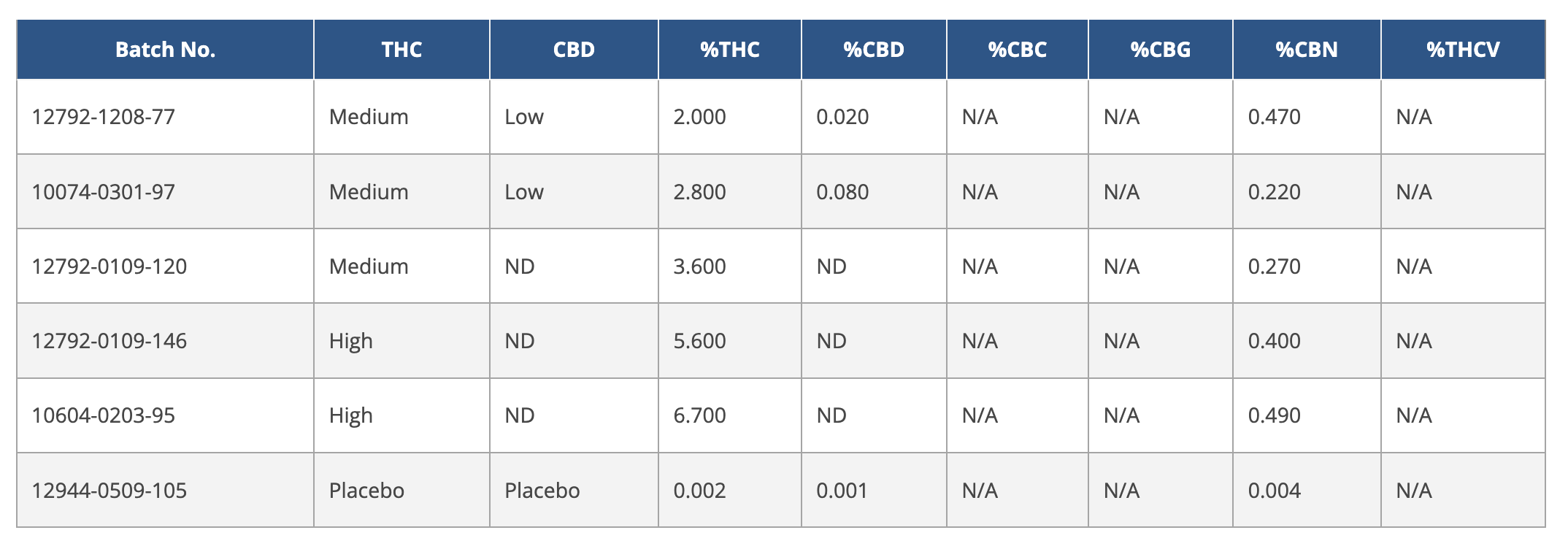
One of the most significant recommendations will benefit clinical researchers who need better, more representative plant material to conduct meaningful research. Since 1968, the University of Mississippi has been the primary supplier of federal research-grade plant material that’s been found to be “genetically divergent from commercially available cannabis” and much closer to hemp, according to one Colorado study in 2019 that looked at 49 cannabis samples, including hemp and materials supplied by farms licensed by the National Institute on Drug Abuse (NIDA). The NIDA lists the products currently available to researchers, offering “high THC” joints with 6.7 percent THC and “very high THC” bulk plant material with THC content of more than 10 percent, without any mention of terpenes and very limited details about lesser cannabinoids. In legal markets, most consumers expect a “high-THC” product to cross the 25-percent threshold, far above what’s offered by federal farms.
While acknowledging the historical limitations of a single supplier, the FDA would expand sources of cannabis for clinical research to any materials “deemed to be of adequate quality by FDA when reviewed as part of an IND [investigational new drug]” application.
The FDA also noted cannabis and cannabis-derived substances are no different from other “botanical raw material, botanical drug substance, or botanical drug product.” The department’s general guidance and recommendations for cannabis are informed by “Botanical Drug Development: Guidance for the Industry,” a guidance document that provides the core principles for conducting clinical research on botanical drugs. The FDA recommends researchers studying cannabis consider the following principles and documents from the United States Pharmacopeia (USP) General Chapters:
- Adequate characterization of each compound via a chemical fingerprint to ensure batch-to-batch consistency.
- Microbiological Examination of Nonsterile Products: Microbial Enumeration Tests (USP General Chapter 61).
- Microbiological Examination of Nonsterile Products: Tests for Specified Microorganisms (USP General Chapter 62).
- Sterility Tests (USP General Chapter 71).
- Elemental Impurities—Limits (USP General Chapter 232).
- Residual pesticide tests (USP General Chapter 561).
- Identification of Articles of Botanical Origin (USP General Chapter 563).
- Microbiological Examination of Nonsterile Products: Acceptance Criteria for Pharmaceutical Preparations and Substances for Pharmaceutical Use (USP General Chapter 1111).
- Validation of Compendial Procedures (USP General Chapter 1225).
In its recommendations, the FDA acknowledged the difficulty in calculating the delta-9 THC content early in the drug development process to qualify a product as hemp or cannabis under the 2018 farm bill. According to the FDA, researchers should not rely on the 0.3-percent delta-9 THC by dry weight standard “when evaluating tetrahydrocannabinols as impurities for the purposes of quality control and application submission.” Instead, the FDA recommends researchers follow the regulations defined by 7 CFR 990 from the Domestic Hemp Production Program (DHPP), which allows for a level of uncertainty.
“For example, if the reported total delta-9 tetrahydrocannabinol content concentration level on a dry-weight basis is 0.35 percent and the measurement of uncertainty is ±0.06 percent, the measured total delta-9 tetrahydrocannabinol content concentration level on a dry-weight basis for this sample ranges from 0.29 percent to 0.41 percent,” the DHPP states. “Because 0.3 percent is within the distribution or range, the sample is within the acceptable hemp THC level for the purpose of plan compliance.”
All THC measurements should be taken before extraction or other manufacturing processes, and all finished products may be considered a Schedule I controlled substance if they contain greater than 0.3 percent delta-9 THC by dry weight.
“We note that, even if the starting materials meet the definition of hemp, intermediates or drug products that contain greater than 0.3 percent delta-9 THC by dry weight may no longer meet the definition of hemp and may be considered Schedule I controlled substances,” the FDA’s recommendations state.
While the FDA’s recommendations do not establish any legal framework for regulation, they do show the department’s “current thinking” on the legal definitions and regulatory controls related to cannabis, providing industry professionals a glimpse of regulations to come.
Comments
Post a Comment